
Unlike most other polymers, nylon is extremely sensitive to moisture. Finding the appropriate balance between too much and too little is essential, yet the need is often overlooked — a leading cause of nylon processing issues and part defects.
Hydrolysis is the chemical breakdown of a compound due to reaction with water. In nylon-based compounds this can adversely affect process conditions and can result in poor product performance. Understanding the basics and the nuances of this chemical reaction between nylon and water goes a long way to eliminating the risk in your nylon-based applications.
Nylons have good thermal and mechanical properties due in part to hydrogen bonding between polymer chains. The hydrogen atom on one polymer chain bonds to the oxygen atom on another polymer chain.
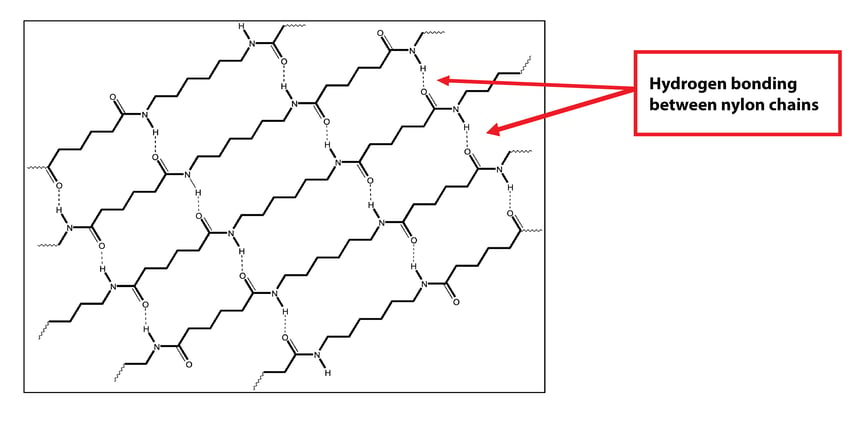 Moisture or water that is absorbed into the nylon polymer also wants to hydrogen bond. The hydrogen in water bonds to the oxygen in the nylon polymer chain while at the same time the oxygen in the water bonds to the hydrogen in the nylon polymer chain. This hydrogen bonded water molecule acts like a plasticizer, allowing the polymer chains to move much more easily.
Moisture or water that is absorbed into the nylon polymer also wants to hydrogen bond. The hydrogen in water bonds to the oxygen in the nylon polymer chain while at the same time the oxygen in the water bonds to the hydrogen in the nylon polymer chain. This hydrogen bonded water molecule acts like a plasticizer, allowing the polymer chains to move much more easily.
If nylon is molded too wet it will drool, flash, or foam as the bonded water molecules are released when the nylon is melted as all the hydrogen bonding is broken. Moisture absorbed onto the surface can generally be removed by drying for 3-4 hours. If the water is absorbed deeply into the polymer chains, more time is needed; sometimes in excess of 24 hours to allow for the hydrogen bonds to be broken and the water molecules to diffuse to the surface.
The above scenario describes moisture absorption - not hydrolysis.
It’s when that existing previously absorbed moisture is not removed that hydrolysis, or the breakdown of molecular weight of the nylon polymer, will eventually occur. However, this is generally not common when nylon pellets simply absorb moisture from the air. The description of effects seen when molding wet resin can be confused with hydrolysis but is often more likely the actual hydrogen bonding between chains - not breakdown of the chains. Breakdown of the nylon polymer chains due to hydrolysis takes a much longer time or a much more aggressive set of conditions.
Let’s look at a practical example:
If a box of pellets is open to the air it will absorb moisture until it hits equilibrium. This takes over 1 year at roughly 23°C at 50% Relative Humidity (RH). Equilibrium is likely when all the hydrogen bonding between water and the nylon chains becomes saturated, i.e. “there is no more room at the Inn...” Notice that we are still not talking about breakdown of the amide bond and the molecular weight is still intact. At this stage, all that has happened is moisture absorption – hydrolysis has not taken place at all. When this moisture absorption does occur it can be seen through data as improved impact strength over time as a result of the plasticizer allowing the polymer chains to move easier and move past each other easier. In fact, it’s not uncommon to see the impact strength continue to increase for up to a year, and then remain stable. But this is for air absorption - now…let’s make the conditions more aggressive.
Let’s place molded bars in water at say 120°C under pressure. In the first few hours the impact strength will increase as with air absorption; but, at some point the impact strength will begin to decrease. This is when hydrolysis happens. In other words, the molecular weight of the nylon is now being reduced as a result of the water breaking the amide bond. The tensile and flex properties will always be in decline - at first due to moisture absorption and the plasticization effect, but later as a direct result of the hydrolytic degradation and breakdown of the molecular weight. This becomes particularly important in applications which require exposure to cooling fluids, e.g. hydrolytic degradation can be seen and verified in an experiment requiring 50% ethylene glycol / 50% water at 135°C by measuring molecular weight. Properties decline from the start of the experiment and after the common test time of 3000 hours we can see a large decrease in molecular weight and a significant reduction in properties often resulting in:
Since moisture can introduce a number of issues, logic seems to dictate that completely drying nylon material would also eliminate the issues. But, this isn’t accurate either – a balance must be struck.
The goal is to maintain the appropriate amount of moisture for a given nylon formulation. Otherwise, you’re trading one set of problems for another.
As mentioned above, the plasticizing effect that moisture in nylon produces also increases flexibility and impact resistance, both desirable characteristics of the material. Over-drying nylon — that is, falling outside of the specified moisture range for drying times and/or temperatures — removes water molecules and their plasticizers, increasing polymer viscosity. Nylon is harder to flow, susceptible to additive burn off, and may oxidize causing the natural polymer to yellow.
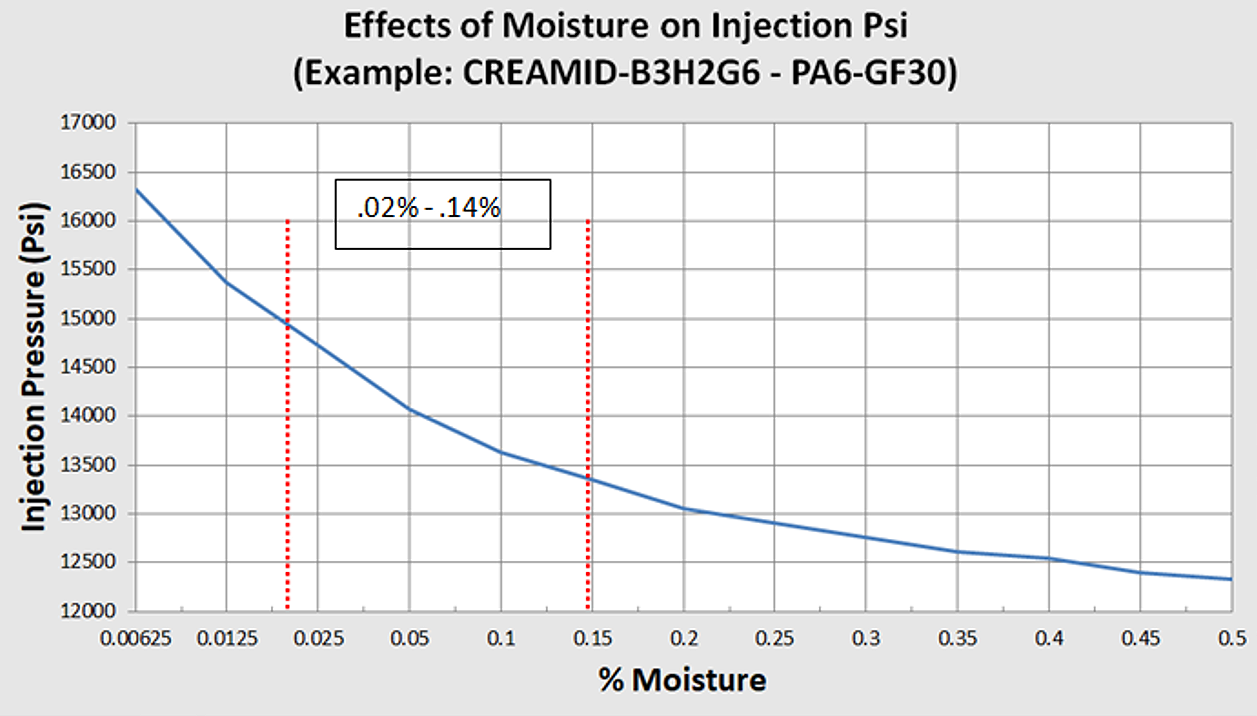
Each nylon material has a unique recommended moisture range listed on its spec sheet. Think of the moisture range as an ideal processing window wherein to transfer the nylon material from the hopper/dryer to the press (injection molding machine).
The benefits of molding nylon within the specified moisture range are many, including:
The caveat to working within moisture ranges is needing to pay attention to additives. For example, unfilled nylon material has a moisture range of 0.02% to 0.20%. The same material containing 30% glass or mineral reinforcement has a moisture range of 0.02% to 0.14%. Why? The additives are not hygroscopic, thereby reducing the range.
Nylon is an extremely versatile material, but it can also present complications where moisture is concerned. An experienced custom compounder like Teknor Apex can help you leverage the benefits of nylon while avoiding hydrolysis and other moisture-related pitfalls, and offer specific solutions to consider like Creamid® P low moisture absorption nylon 6. Click the button below to learn more.